They could save hundreds of lives a year in the USA alone, but they can’t be bothered to do the preventative test.
And that, despite the FDA announcing in 2006 that they were ushering in a new era of “personalized medicine” (aka. precision medicine) where it would be pronounced negligent malpractice NOT to test a patient before treatment, to ensure they could tolerate it.
I thought it was too good to be true at the time, though I wrote about it enthusiastically. As it has emerged, it’s more about choosing the right treatment and little at all to do with safety testing.¹
See, I’ve been writing for years that doctors try to treat an “average” person, even though there is not one single average person on this planet. We are NOT all the same (thank God) and we all vary in many different parameters. Someone might be of average height but that doesn’t mean they have an average weight or, even more problematical, that they have the same detox mechanisms for removing poisons.
Take chemo: we all know it can be pretty grim. But people vary. Someone like me could probably take massive doses and laugh it off, whereas somebody else might be brought to death’s door within a week.
I’m thinking now of one particular agent: fluorouracil (5-FU). It came out in my day at med school (1962). It is given IV to treat colorectal cancer, esophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts.
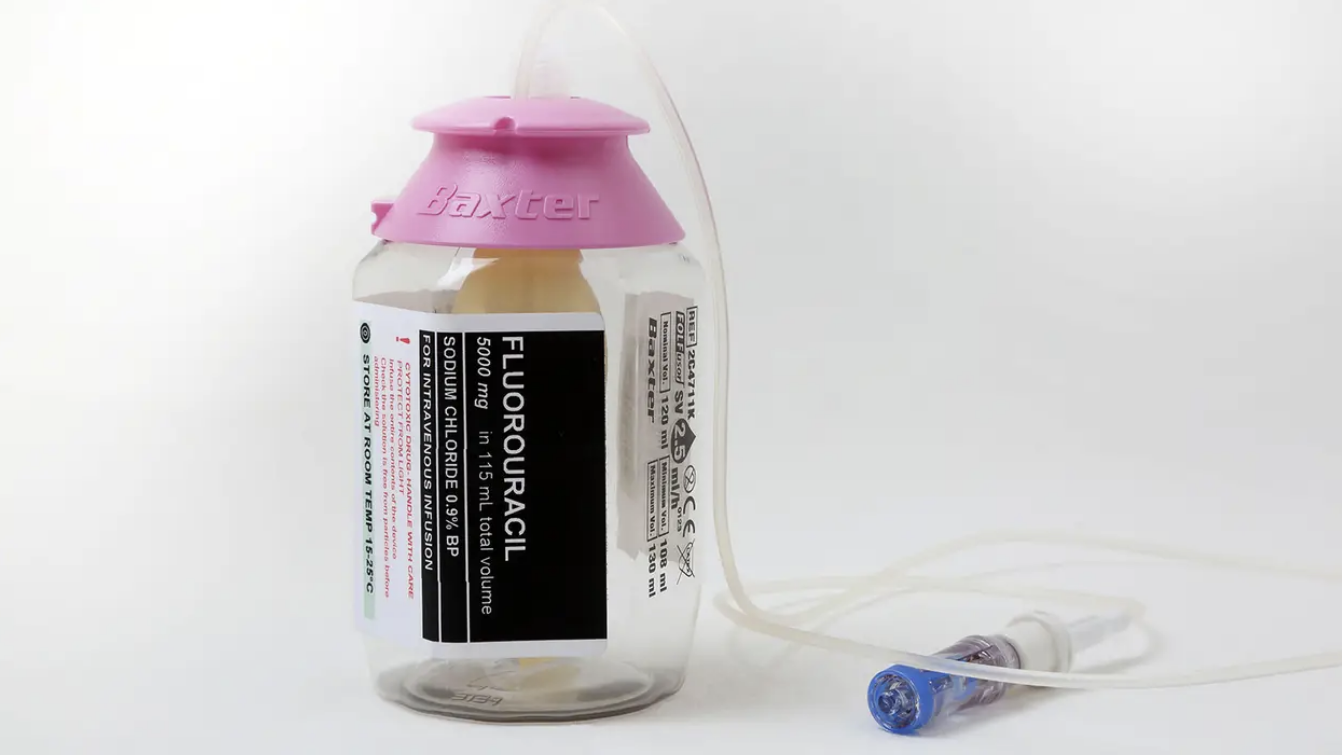
The BIG problem is there is very little difference between the minimum effective dose and maximum tolerated dose (a very narrow safety window) and the drug exhibits marked individual pharmacokinetic variability. Therefore, an identical dose of 5-FU may result in a therapeutic response with acceptable toxicity in some patients and unacceptable, possibly life-threatening toxicity in others.
If you are one of the unlucky individuals who can’t tolerate it, you might end up dying in misery, even agony, with severe diarrhea, nausea, skin peeling away in sheets, terrible sores in the mouth that prevent eating and drinking, and eventually, prevent speaking. Finally, the liver and kidneys fail. That’s you… now an EX-patient, an EX-person.²
What a horrible way to go. You wouldn’t want that for someone in your care, surely? And if there was a simple test to weed out individuals who cannot tolerate 5-FU, you would surely test everyone first, before prescribing it, right?
There is such a test and it’s called DPYD (dihydropyrimidine dehydrogenase deficiency testing). Doctors can order the test and get results within a week. If the patient is one of the unlucky few, then there is the chance to either switch drugs or start with a much lower dose.
Well, as final proof of just how callous US oncologists can be, very few (3%) order the DPYD test.³ Note that pretreatment DPYD testing is in use throughout most of Europe, including the UK. The pathetic excuse is that U.S. cancer treatment guidelines, issued by the National Comprehensive Cancer Network (NCCN), “don’t recommend preemptive testing.”
It doesn’t wash with me because the test IS available in the USA and doctors are supposed to think about consequences for themselves.
Look, I’m hoping none of you ever get this stuff or are presumed to need it. I’m merely expostulating on how bad the practice oncology has become.
Chemo is “no picnic” for anyone, as a piece in MedPage Today remarked. But if you are deficient in the critical enzyme (DYP), the outcome could be lethal. Instead of (hopefully) extending your life a few more months or years, you could be dead within a week.
DYPD patients essentially overdose because the drugs stay in the body for hours rather than being quickly metabolized and excreted. The drugs kill an estimated one in 1,000 patients who take them—that amounts to hundreds a year—and severely sicken or hospitalize one in 50. It’s a bad drug.
The FDA added new warnings about the lethal risks of 5-FU to the drug’s label on March 21, 2024. However, it did not require doctors to administer the test before prescribing the chemotherapy. The agency has said it could not endorse the 5-FU toxicity tests because it’s never reviewed them.
But that’s total hooey. The FDA does not at present review most diagnostic tests, never mind this critical one!
“FDA has responsibility to assure that drugs are used safely and effectively,” Daniel Hertz, PharmD, PhD, of the University of Michigan College of Pharmacy in Ann Arbor. The failure to warn, he said, “is an abdication of their responsibility.”
British and EU drug authorities have recommended the testing since 2020. A small but growing number of U.S. hospital systems, professional groups, and health advocates, including the American Cancer Society (ACS), also endorse routine testing. Most U.S. insurers, private and public, will cover the tests, which Medicare reimburses for $175, although tests may cost more depending on how many variants they screen for.
In European hospitals, the practice is to start patients with a half- or quarter-dose of 5-FU if tests show a patient is a poor metabolizer, then raise the dose if the patient responds well to the drug, without any adverse reactions. Advocates for this approach say American oncology leaders are dragging their feet unnecessarily, and harming people in the process. KILLING, actually (my emphasis).
“Anybody who’s had a patient die like this will want to test everyone,” said Robert Diasio, MD, of the Mayo Clinic in Rochester, Minnesota, who helped carry out major studies of the genetic deficiency in 1988.
We can only hope that mandatory testing is not far away. Then providers will be bullied into doing it right.
Plavix Has No Effect On 25% Of Patients!
Oncology is not the only area in medicine in which scientific advances, many of them taxpayer-funded, lag in implementation. For instance, few cardiologists test patients before they go on Plavix, a brand name for the anti-blood-clotting agent clopidogrel, although it doesn’t prevent blood clots as it’s supposed to in a quarter of the 4 million Americans prescribed it each year. In 2021, the state of Hawaii won an $834 million judgment from drugmakers it accused of falsely advertising the drug as safe and effective for Native Hawaiians, more than half of whom lack the main enzyme to process clopidogrel.
Just another instance of Big Pharma lying to suit themselves, even though it puts patients at risk. Nothing new!
To Your Good Health,Prof. Keith Scott-Mumby
The Official Alternative Doctor
Grim reading for Easter maybe. Still, have a nice weekend!

SOURCES:
- Published July 22, 2010. N Engl J Med 2010;363:301-304
- https://www.medpagetoday.com/hematologyoncology/chemotherapy/109367
- JCO Oncol Pract. 2022 Jun;18(6):e958-e965. doi: 10.1200/OP.21.00874. Epub 2022 Mar 3




