This is an interesting story in its own right (well, shocking). But colchicine is a worthy drug and you may have cause to get your doctor to try it. This has been the fight:
In June 2023 the U.S. FDA approved a proprietary version of an age-old drug colchicine as the first anti-inflammatory drug for the prevention of heart attacks and strokes among people with established heart disease or multiple risk factors (marketed as Lodoco). One recent review reported that inflammation is a better predictor of future cardiovascular events and death than elevated cholesterol. So, the availability of a medicine that addresses inflammation is good news.
But the dark side to this approval is about money. They are now charging $20 a pill; more than 20 times the previous price! What a scam, with the FDA complicit in the crime. Colchicine is not a new drug. I myself prescribed it in my early days for patients with gout. It is made from the autumn crocus (Colchicum autumnale) and has been in use for millennia. It was used to treat gout in ancient Greece over 2,000 years ago and there is a report of its effectiveness in the famous Egyptian Ebers Papyrus (1500 BC).
 The pretty autumn crocus
The pretty autumn crocus
Now it’s been “discovered” as a new treatment for inflammatory heart disease. It just shows you how really crooked is the “science” of drug research.
The model is simple: find something you know works, preferably historically so. Modify it and patent it. Make billions in profit, meanwhile attacking the “unproven” original remedy!
Over the last few years, a significant number of studies have documented that colchicine reduces the risk of heart attacks and strokes among people with established heart disease. One large study published in 2019 followed heart disease patients for nearly 2 years and tabulated how many people died from cardiovascular causes, were resuscitated, experienced cardiac arrest, had a heart attack or stroke, or needed urgent hospitalization.1
In comparison to those randomly assigned to take a placebo, those taking colchicine were 23% less likely to experience one of these outcomes. That’s fairly significant.
In a 2020 study, among patients with chronic coronary disease, most of whom were already receiving proven secondary prevention therapies, 0.5 mg of colchicine once daily resulted in a 31% lower relative risk of cardiovascular death, spontaneous myocardial infarction or ischemic stroke.2
Now the twisted part… Although colchicine has been used successfully for centuries, it had not been patented until relatively recently. Back in 2006, the wily FDA created the “unapproved drugs initiative” for older generic drugs, like colchicine. Companies that invested in these evaluations were rewarded with the opportunity to patent the molecules (which strictly speaking is not within the FDA’s purview).
Immediately the pharmaceutical companies began doing clinical studies on these older medications. Given the timing, we believe the primary goal was to bring them to market as high-cost patented brand-named products. Of course! What other goal does Big Pharma ever have?
Then in 2009, the FDA allowed URL Pharma to bring colchicine to market under the brand name Colcrys at a new dose of 0.6 mg per pill. Under the Orphan Drugs Act, they were given 3 years of market exclusivity at this dose for the treatment of gout. As part of these agreements, unapproved single-ingredient colchicine could not be sold in the U.S. and disappeared from the market. During the decade following the price increase, there was a 27% reduction in the use of colchicine among gout patients who needed it. A useful older drug swept away, to allow this egregious grab!
The FDA and the public soon recognized that the unintended consequences of the initiative also included drug shortages and higher prices to treat gout. With these new approvals, the cost of colchicine accelerated from pennies to about $5 per pill. The rheumatology community and other groups were extremely upset when their patients could no longer obtain generic colchicine at what many prescribers viewed as a fair price.
They simply suppressed and eliminated generic colchicine, to help along their crooked pharma buddies (who pay the wages at the FDA, remember). In other words, profit over people.
Agepha Pharma today holds eight patents on Lodoco for preventing heart disease or stroke. Now that the periods for exclusive marketing of Colcrys have expired, the price has come back down. Although the retail price remains around $5 per pill, large chain pharmacies sell it for less than $1/pill. Colcrys still remains FDA-approved for the treatment of gout at a dose of 0.6 mg per pill and generics are still outlawed.
Robert M Kaplan, PhD, and Michael Wiseman MD wrote extensively about this scam for MedPageToday’s second opinion and made their views pretty clear. Nevertheless, the authors had to do their CYA and added the following note of circumspection:
To be clear, we are strong supporters of the search for safe and effective medications. We recognize that the process requires significant investment. But colchicine has a long history of use and has established its value in several areas of medicine… For those with inadequate resources or lack of health insurance, allowing this well-established generic medication to become patented and hence unaffordable to some has the potential to produce significant harm. We hope Congress and the FDA will take this issue seriously.3
Meanwhile, Agepha, the manufacturers of Lodoco, just released a retail price for Lodoco of $621 for a 30-day supply — nearly $21/pill. Lodoco can cost as much as four to six times more than Colcrys. Yet, Colcrys and Lodoco are essentially the same drug. Both have only one active ingredient — colchicine. The only difference is a tiny variation in the dose. Colcrys has 0.6 mg of colchicine, while Lodoco has 0.5 mg — a difference that could be considered of no clinical consequence.
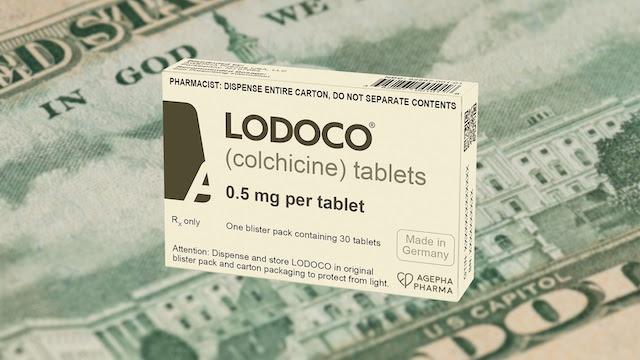
Doctors who want to use colchicine to prevent heart disease will in theory be able to legally write off label prescriptions for the lower cost Colcrys. However, insurance companies, the bane of the medical profession, may deny payment because the dose of 0.6 mg was not approved for this indication (heart disease prevention).
If you feel the need, you may be able to get this humble drug at GoodRx, who are offering prices ranging from $9.99 for a 30 day supply of 0.6 mg/30 tabs at Costco, to a high at Duane Reede of $49.
Or go to a Mexican online pharmacy and buy Sixol, 1mg (that’s double the dose available in the US). But be prepared to be harassed by government friends of Big Pharma for sidestepping the money grinder!
To your good health,
Prof. Keith Scott-Mumby
The Official Alternative Doctor
References:
- https://www.medpagetoday.com/meetingcoverage/aha/83388
- November 5, 2020. N Engl J Med 2020; 383:1838-1847 DOI: 10.1056/NEJMoa2021372
- https://www.medpagetoday.com/opinion/second-opinions/106756?xid=nl_secondopinion_2023-10-15&eun=g883510d0r




