As part of my ongoing DETOX WEEK, let’s take a look at how toxins impact our body metabolism. I don’t think you’ll find it too onerous…
Toxicology is one area of medicine about which clinical ecologists (like me) are not in conflict with their more conventional colleagues. We share the same issues, pursue the same phenomena and agree on similar therapeutic approaches. Toxicologists tend to veer in the direction of epidemiological effects, studying whole populations, whereas the clinical ecologist takes it more patient by patient, but that’s the only real difference.
Toxicology has been around a surprisingly long time. Primitive peoples used natural poisons for hunting. Indeed the word toxicology comes from toxicos, the bow from which poison arrows were flung. The ancient Greeks and Romans made a special study of poisons, although more in connection with political assassinations than the pursuit of science. The Persian King Mithridates was so afraid of being poisoned that he took a regular cocktail of known poisons to accustom his body to their effect so that they would no longer work on him. From his name we get the word mithridate.
The prolific use of poisons for getting rid of ‘inconvenient’ people led to a treatise by the Jewish physician/philosopher Moses Maimonides (1135-1204) from Cordoba (Spain) entitled Poisons and their Antidotes. It summarized all knowledge of poisons at that time.
 Maimonides
Maimonides
The Italian fifteenth-century Borgia family were infamous poisoners. Lucrezia’s name, in particular, achieved evil notoriety for her nefarious use of chemicals to ‘alter’ history.
Toxicology finally adopted a more formal scientific footing, and today we are concerned almost entirely with environmental hazards and unintentional harm done to human beings. Around 4,000,000 man-made chemicals have been described in scientific literature since 1965. Something like 6,000 new chemicals are added to the list every week and at least 70,000 – 80,000 are currently in production. Only a fraction of this toxic load has been adequately tested for the long term effects on human health.
We meet chemicals in the air, our food, water supplies and by direct contact. Medical drugs add their share, and even the clothes we wear and the fabric of our homes are mostly artificially made, needing many complex chemical precursors. Some of these emit toxins long after being installed in the home. It is a fact of life in the modern world that indoor pollution can be just as bad, or worse, than the outdoor kind.
The cumulative effect of all these substances may create a total body burden that triggers chemical sensitivity in certain individuals. In the late 1970s Dr. E.C. Hamlyn coined the term ‘human canary’ to describe such people – they are a warning to us all that we are going to be ill if we continue as we are, in much the same manner that canaries used to warn miners of impending gas danger. Unfortunately, no one seems to be heeding these canaries.
Most studies done on humans to date have been concerned predominantly with acute massive exposures suffered by workers in industrial settings, but clinical ecologists have been gathering case studies steadily to show that chronic exposure to levels commonly thought to be ‘safe’ are compromising people’s health and may turn out to be a more important hazard in the long term.
Multifactorial
The effects of chemical exposure are dependent upon a number of factors, principally:
• the amount and biological activity of the compound
• length of the exposure time
• genetic factors
• biochemical individuality
• the total stress load
• age and sex
• previous exposures
• nutritional factors
The resulting problems can be complex, depending on the target organs involved. Misdiagnosis and missed diagnosis are the norm. Safety levels are misleading, since they are based on averages. Some individuals will react to far lower levels than would affect the majority.
The way the body disposes of unwanted and toxic compounds (xenobiotics) we call detoxification. In fact, the metabolic pathways discussed here, by which chemicals are altered, inactivated and removed from the body, don’t always result in a less poisonous end-product. A better term, therefore, is biotransformation.
There are several pathways involved. The subject is a vast and burgeoning one; the information given here is necessarily selective.
Metabolism Of Toxic Compounds
To get rid of a toxin effectively it is most important that the body turns it into something soluble in water. At that point the substance or its metabolites (breakdown products) can be removed via the kidneys, sweat, bile and other fluids. There are two principal routes by which the body does this. In what we call Phase I detoxification the molecule is altered by enzymes in a variety of ways, each process assisted by a specific enzyme. These enzymes are found in the microsomes of most cells.
The most important of these enzyme pathways is the monstrous “cytochrome P450 system”, also called the multi-function oxidase system (MFO). Under its influence oxygen is added to the toxic molecule, converting a hydrogen atom in the molecule into a hydroxyl group (hydrogen and oxygen). The opposite effect, known as reduction, means that hydrogen is added. Both effects can knock out the toxicity of a molecule.
Two other methods to note are: adding a water molecule (2 hydrogen and 1 oxygen atoms), called hydration and knocking out halogen atoms, such as chlorine, called dehalogenation.
Note that magnesium is essential for Phase I actions, as is a complex co-enzyme called nicotinamide adenine dinucleotide (NAD+), a derivative of vitamin B3. This is why your NMN (Nicotinamide Mononucleotide) molecule is useful for extending life!
Vitamin C and zinc are also said to help, and possibly other nutrients as well. This is why vitamin and mineral supplements can be so vital for allergics and poor metabolizers.
Phase II detoxification is carried out differently. Here extra groups are stuck on to the basic molecule. These change its character and render it harmless and more soluble. We call this process conjugation. An example is sulphation, the addition of a sulphate group (-SO3).
Thus toxic benzene is converted to equally toxic phenol (carbolic acid), which in turn is altered into relatively harmless phenol sulfate. Here’s a graphic representation of phase I and phase II changes:
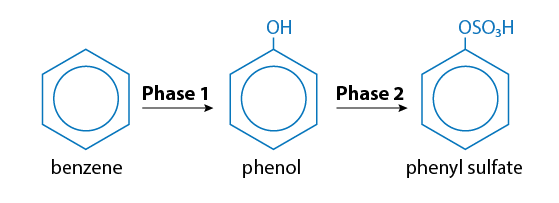
The enzyme in this case is sulphonyl transferase. Phenol sulphonyl transferase may have great importance for food intolerance since it has become clear that a number of foods contain numbers of phenolic compounds.
Side Routes
These optimal biotransformation pathways can be blocked for a number of reasons. Certain vitamin and mineral deficiencies could do it; magnesium, for example, has been mentioned. Overload can have the same effect. As the total quantity of xenobiotics increases, we can produce more of the relevant enzyme, up to a point (this is called enzyme induction). But eventually we pass the equalization point and the body can no longer cope.
When the basic system is no longer capable of keeping pace, ‘alternative’ toxic metabolites may be chosen which may be more stable and can’t easily be brought back into the enzyme pathways and broken down.
Some of these ‘alternatives’, for example epoxides, are capable of causing serious tissue and gene damage, even cancer.
A Cause of Fatigue
Less serious, perhaps, but troublesome nevertheless is the alternative pathway that yields chloral hydrate. At times of overload, this chemical can build up. It is one of the ingredients of the classic ‘Mickey Finn’. If this build-up occurs the patient will begin to feel very fatigued and ‘spacey’. These are symptoms that overload patients will recognize.
Candida, incidentally, is capable of producing alcohols and aldehydes, which also add to the overload of these detoxification pathways.
The diversity of routes for xenobiotics and the consequences that they may have for the organism can be represented diagrammatically, as shown in this figure.
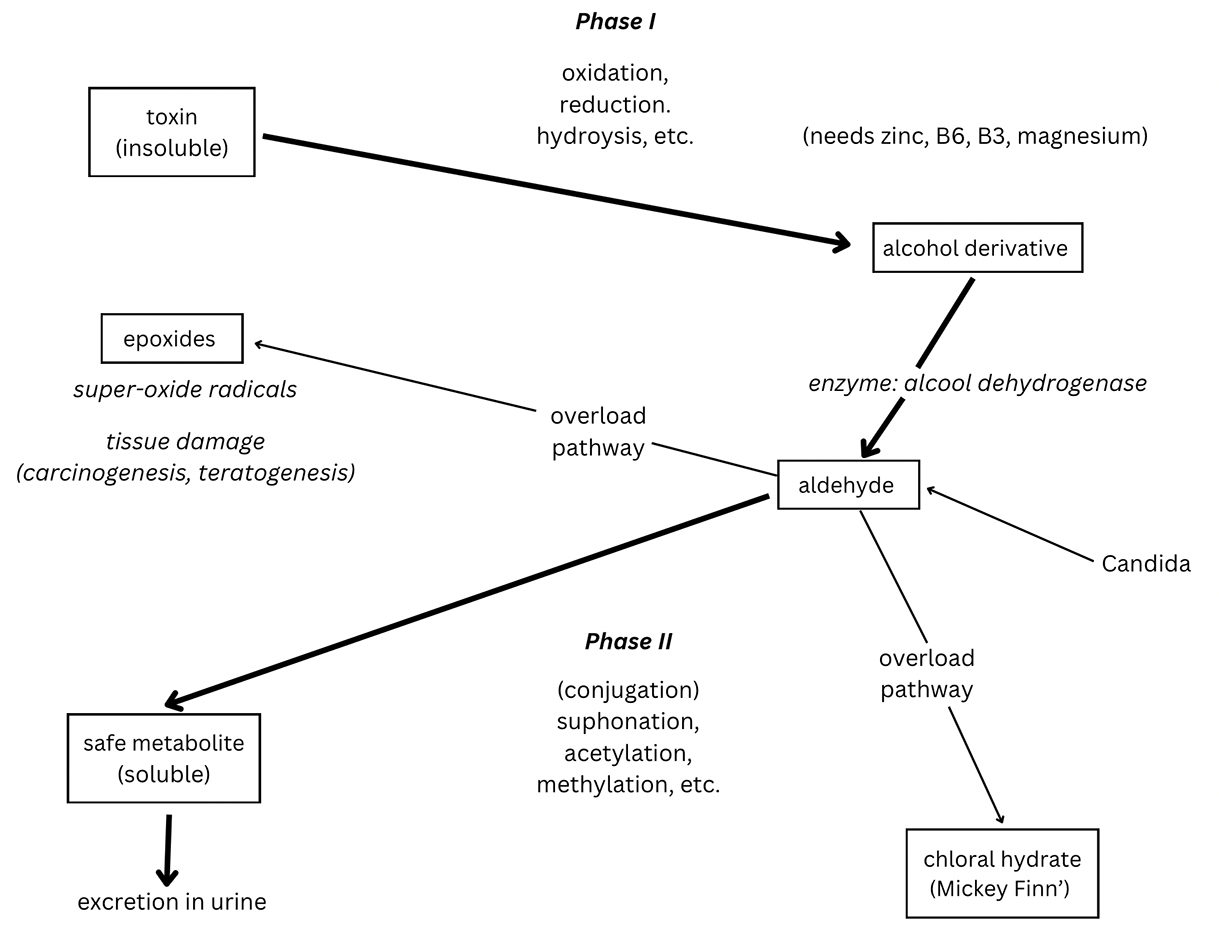 Detoxification pathways and overloads
Detoxification pathways and overloads
Fast And Slow Metabolizers
There is a sulphoxidation reaction test the can detect the amount of sulphoxide in the urine following a loading dose of a suitable test substrate.
Those who don’t handle xenobiotic chemicals very well produce less metabolite output to the urine. We can call them ‘slow metabolizers’. Preliminary results show that the white European population has less than 20 per cent slow metabolizers.
A genetically-determined ability to metabolize in this way could be an important factor in a person’s response to toxic environmental chemicals, heavy medical interventions like chemo, and could determine whether he or she will ultimately contract cancer.
The implication of all this is startling, to say the least. If it turns out to be correct it means that many diseases may have a basis in chemical overload. For example, the incidence of Parkinson’s disease (PD) is found to have a an extraordinarily exact correlation with areas of high pesticide use and also affects a larger-than-expected percentage of slow metabolizers. Could PD be an ecological illness?
Work continues.
To Your Good Health,
Prof. Keith Scott-Mumby
The Official Alternative Doctor
NEXT: One surefire way to get all this gunk out of your body…




